We are known for our Innovation.
There is tremendous value in Investing in Research & Development. Even though our early investment in R&D started few years ago, this makes us really technically differentiable. Our core strength is to develop complex generic product within defined timeline.
Our capabilities span the development of differentiated products, such as liposomal products, micro emulsions, lyophilized injections, osmotic pumps, besides developing controlled release dosage forms.
Our scientists work closely with our business development team to generate innovative concepts and ideas, exploiting both market needs and synergies across therapeutic areas. We invest around 5 per cent of our revenues annually in research.
We have more than 100 formulators & analyst working in multiple R&D centers equipped with cutting-edge enabling technologies for research. Our scientists have expertise in developing generics, difficult to make technology intensive products & Active Pharmaceutical Ingredients (APIs).
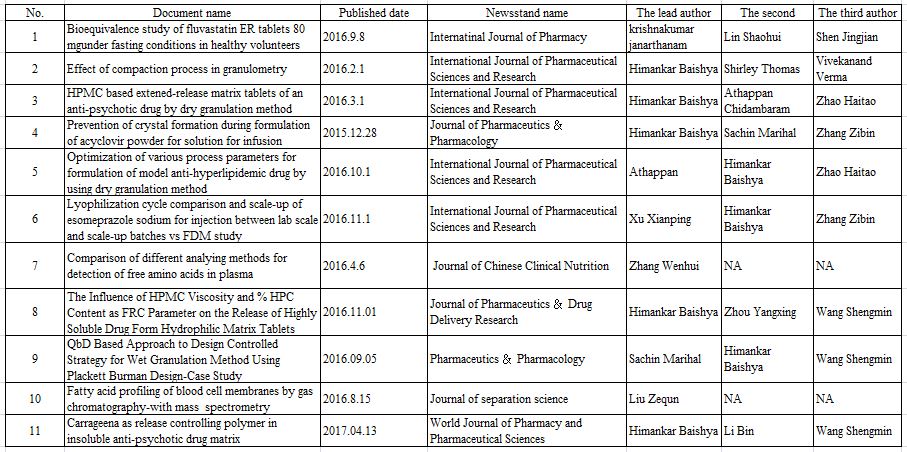
Within this short time span, we have developed expertise and gathered experience in performing pharmacokinetic and bioequivalence studies to facilitate the introduction of generic or branded generic drugs into the international market.
Apart from the involvement of developing various specialized products for USA, Europe, China and Other regulatory and non-regulatory market, our R&D Scientist also involve in many tech-transfer and CMO activities. With this continuous systematic technical support from R&D team, Sciecure will earn good revenue as of now and near future.
Analytical
Analytical Department has well-resourced laboratories and skillful analyst to perform the analyses under cGMP/GLP conditions for both domestic and international regulatory requirements. Laboratories are well equipped with state-of-the-art instruments with the well-trained operators to use and maintain them under the validated conditions.
We develop and validate test methods to regulatory markets including USFDA, MHRA, TGA, China and many others. Test methods cover a wide variety of samples come from API, raw materials, finished products, stability and cleaning studies. Method validations are performed following ICH and other regulatory guidelines.
We transfer test methods to/from our laboratory based on approved protocols. We can handle all the test requirements for project transfer, technology transfer or site transfer. We also under take formulation development testing from laboratory trials to large scale.
We work closely with quality control and other partner laboratories providing training, protocols and documentation to qualify partner laboratories to perform sample testing. Our analysts undergo internal and external training from in-house and professional trainers to advance their knowledge and skills.
Our stability lab consists of over 12 chambers including 8 walk-in chambers and a photolysis chamber capable of storing samples at conditions required for Zone I, II, III and IV. Chambers are equipped with continuous monitoring, alarms and automatic messaging system for emergencies and adequate backup system for any break downs.
Lab equipment includes:
High Performance Liquid Chromatography (HPLC)
High Performance Liquid Chromatography coupled with Mass Spectroscopy (HPLC-MS)
Thin Layer Chromatography (TLC)
Ion Chromatography (IC)
Gas Chromatography (GC)
Dissolution Apparatus 1,2 and 3
UV/VIS Spectroscopy (UV)
Infrared Spectroscopy(IR)
Malvern Particle Size Analyser
Karl Fischer Titrator
Micro, Semi-micro and Analytical Balances


Synthesis
There are three laboratories for the synthesis, performing independently developing and optimization of API synthesis process from gram grade to kilogram grade with the advanced equipment/instruments. By cooperating with workshop, we can conduct pilot trial, pivotal, and submission batch as well as commercial production.
Currently we are proceeding six projects for the target markets like China, Europe and USA. As a new pharmaceutical excipients, one of them has been granted the certificate for the clinical trials by CFDA. In addition, there are several APIs entitled for the production certificates, and two have now been prepared for the production.
Besides developing autonomously, we has collaborated with many companies to develop the new projects and supplied the best quality and value APIs to both our company and enterprise.
Formulation
Sciecure is an innovative research and development based pharmaceutical company. We are engaged in the development of generic and novel drug delivery systems that provide innovative solutions to the current global healthcare needs. Our research and development facilities are situated at Beijing, China, and New Jersey, USA.
Sciecure has a modern and well-equipped R&D Centre, ranked amongst the best in the country. At Sciecure we have a team of highly trained and qualified research scientists, who all hired based on their technical expertise from across the world, engaged in various product development works. Our development strategy is aligned with current regulatory requirements for drug development that includes Quality by Design (QbD) approach like Design of Experiments (DoE) and other concepts.
Our expertise includes but not limited to osmotic drug delivery systems, immediate release dosage forms, extended release dosage forms, drug loaded pellets and injectable dosage forms (solution, emulsions and dispersion).
Today, Sciecure with the research facilities and a global presence, is all poised to carve a niche for itself in the international pharmaceutical arena. It is well set on an exciting growth phase in all directions.
R&D Thesis